




What is the activation energy (Ea)?
Activation energy, also known as threshold energy, was introduced by Arrhenius in 1889 to define the energy barriers that need to be overcome for a chemical reaction to occur.Activation energy can be used to represent the minimum energy required for a chemical reaction to occur. The activation energy of a reaction is usually expressed as Ea, in kilojoules per mole (kJ/mol).For first-order reactions, the activation energy represents the height of the potential barrier (sometimes referred to as the energy barrier). The magnitude of activation energy can reflect the difficulty of chemical reactions occurring.
Activation energy is a field of kinetic research that can be used to calculate the relationship between reaction rate and the concentration of reactants and catalysts. The study of chemical kinetics is an important means of studying the time required to complete a chemical reaction process, the influencing conditions, and the mechanisms by which this process is achieved.
How to measure activation energy using a chemical adsorption instrument?
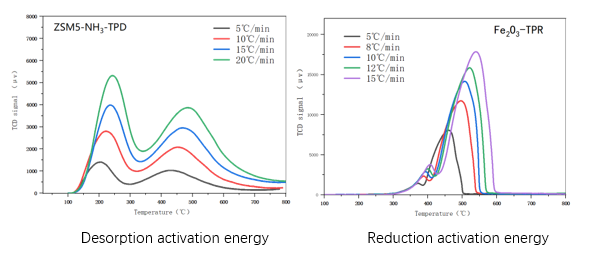
In the study of reaction kinetics used in the PCA1200 chemical adsorption instrument, the desorption activation energy and reduction activation energy can be obtained separately, namely TPD (temperature programmed desorption) and TPR (temperature programmed reduction) research processes. The testing methods for desorption activation energy and reduction activation energy are consistent, that is, using the same catalyst and under equal amounts and conditions, changing the heating rate from small to large at least three times, and then plotting 2 pairs of 1/as a straight line, which can be obtained from the slope.
contact
Be the first to know about our new product launches, latest blog posts and more.Any question or request?
Click below, we’ll be happy to assist. contact